Torr Specs by Vacuum Conditions and NASA Nonsense
No Space Suit Made by NASA Can Withstand The Vacuum of Outer Space
What is a Torr?
The torr (symbol: Torr) is a non-SI unit of pressure defined as 1/760 of an atmosphere. It was named after Evangelista Torricelli, an Italian physicist and mathematician who discovered the principle of the barometer in 1644.
Torricelli attracted considerable attention when he demonstrated the first mercury barometer to the general public. He is credited with giving the first modern explanation of atmospheric pressure. Scientists at the time were familiar with small fluctuations in height that occurred in barometers. When these fluctuations were explained as a manifestation of changes in atmospheric pressure, the science of meteorology was born.
Over time, 760 millimeters of mercury came to be regarded as the “standard” atmospheric pressure. The unit of barometric pressure (one millimeter of mercury, also written as 1 mm Hg) was named in honor of Torricelli.
In 1954, the definition of atmosphere was revised by the 10e Conférence Générale des Poids et Mesures (10th CGPM) to the currently accepted definition: one atmosphere is equal to 101325 pascals. The torr was then re-defined as 1/760 of one atmosphere. This change in the definition of “torr” has been a source of confusion ever since.
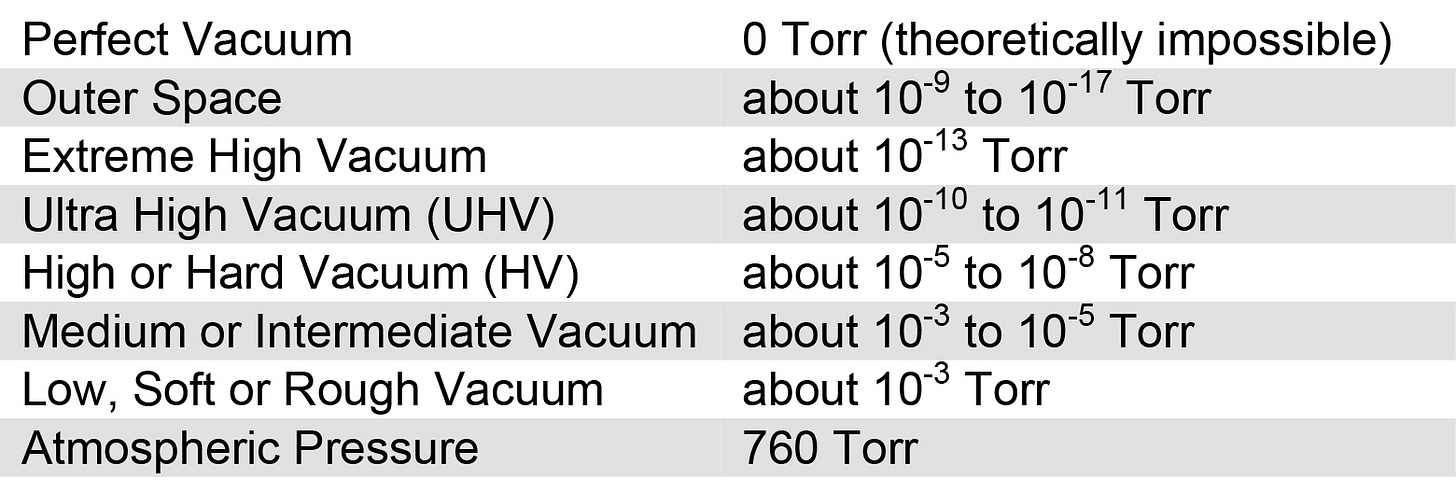
Torr Specs by Vacuum Conditions:
Vacuum quality is subdivided into ranges according to the technology required to achieve it or measure it. These ranges do not have universally agreed definitions (hence the gaps below), but a typical distribution is as follows:
Atmospheric Pressure 760 Torr 101 kPa
Low Vacuum 760 to 25 Torr 100 to 3 kPa
Medium Vacuum 25 to 1×10-3 Torr 3 kPa to 100 mPa
High Vacuum 1×10-3 to 1×10-9 Torr 100 mPa to 1 µPa
Ultra-High Vacuum 1×10-9 to 1×10-12 Torr 100 nPa to 100 pPa
Extremely High Vacuum 1×10-12 Torr 100 pPa
Outer Space 1×10-6 to <3×10-17 Torr 100 µPa to <3fPa
Perfect Vacuum 0 Torr 0 Pa
Low vacuum, also called rough vacuum or coarse vacuum, is vacuum that can be achieved or measured with rudimentary equipment such as a vacuum cleaner and a liquid column manometer.
Medium vacuum is vacuum that can be achieved with a single pump but is too low to measure with a liquid or mechanical manometer. It can be measured with a McLeod gauge, thermal gauge or a capacitive gauge.
High vacuum is vacuum where the MFP of residual gases is longer than the size of the chamber or of the object under test. High vacuum usually requires multi-stage pumping and ion gauge measurement. Some texts differentiate between high vacuum and very high vacuum.
Ultra-high vacuum requires baking the chamber to remove trace gases, and other special procedures.
Deep space is generally much emptier than any artificial vacuum that we can create.
Perfect vacuum is an ideal state that cannot be obtained in a laboratory, nor even in outer space.
Vacuum Examples
Vacuum Cleaner approximately 80 kPa (600 Torr)
Liquid Ring Vacuum Pump approximately 3.2 kPa (24 Torr)
Freeze Drying 100 to 10 Pa (1 to 0.1 Torr)
Rotary Vane Pump 100 Pa to 100 mPa (1 Torr to 10−3 Torr)
Incandescent Light Bulb 10 to 1 Pa (0.1 to 0.01 Torr)
Thermos Bottle 1 to 0.1 Pa (10−2 to 10−3 Torr)
Near Earth Outer Space approx.. 100 µPa (10−6 Torr)
Cryopumped MBE Cham. 100 nPa to 1 nPa (10−9 Torr to 10−11 Torr)
Pressure on the Moon approx.. 1 nPa (10−11 Torr)
Interstellar Space approximately 1 fPa (10−17 Torr
ImpossiBALL Jupiter
Jupiter is said to be a giant ball of gas, with no solid surface. It is allegedly composed of the very light gases, hydrogen and helium. Because of dispersion, Entropy and the Second Law of Thermodynamics, we know gas will always disperse to fill any vacuum. Jupiter cannot be a ball of gas and still remain intact against the near perfect vacuum of Outer Space unless the entire surface of Jupiter has a hard shell container. Jupiter is not a ball of gas.